

Redox potential measurement – how to avoid the most common mistakes?
Redox potential (oxidation reduction potential), along with pH value, is one of the most common process variables in industrial and municipal water treatment plants, as well as in drinking and pool water monitoring systems. In the following post, we discuss its theoretical and practical aspects, including solutions to the most common mistakes. Read on!
Redox potential – what is it? Redox potential definition
Redox or ORP (Oxidation Reduction Potential) is a measure of a liquid's potential to undergo a chemical reaction known as oxidation or reduction. Redox potential controls any oxidation reaction or reduction reaction. It is also an indicator of water quality.
We encounter redox reactions every day without consciously noticing them. Examples include:
-
iron rusting
-
copper forming a patina coating
-
blackening of silver
Stability of the redox potentials means that well-defined oxidizing or reducing conditions prevail.
The redox potential is a measure of electrochemical potential or electron availability within these systems. Electrons are essential to all inorganic and organic chemical reactions.
Redox potential unit
The redox value is given in milivolts (mv) releative to a standard hydrogen electrode.
Redox potential meter
The redox potential is measured by dedicated electrodes for measuring redox potential. It is usually platinium electrode with saturated calomel electrode as a reference.

Iron rusting – one of the redox reactions
Drift of redox potential readings
Redox reference electrodes tend to drift. This intensifies as they are used, eventually leading to the need to replace the device.
However, their service life can be quite long if they do not become damaged or affected by a chemical agent.
In pure water, the redox potential drifts both ways as it reacts to dissolved oxygen concentration, trace amounts of impurities or the presence of solution.
Redox potential measurements – the most common errors
A change in the value of the redox potential can be caused by various factors, including numerous errors. The most common of these are discussed below.
Reference electrode error
Slope changes do not occur with redox electrodes. If false measurements occur, they are most often caused by a dirty (contaminated) electrode surface. This can be easily remedied in this case by cleaning the electrode (distilled water, wiping the electrode metal with fine polishing powder).
The reaction of gold and platinum electrode metals depends on their previous operation and preparatory treatment. If there is a change from a medium with oxidizing properties to a medium with reducing properties or vice versa, a longer settling time before reaching a constant potential may be acceptable.
It is not possible to give universal guidance, since the method of preparation depends on the type of measurement medium. If necessary, carry out your own tests and perform preparatory processing based on them.
Calibration – the frequency of calibration of the pH and redox sensor
If there is an offset in the measured value, single-point calibration can be performed. In single-point calibration, the electrode zero point is redetermined using a buffer solution. The adjustment to the new zero point is made automatically in the transmitter after the calibration process is started.
Critical impacts on the reference system
Malfunction of the reference system, can be caused by:
-
moisture in the cable
-
damaged contacts
-
semiconductive layer not removed
-
short circuit in the connector
-
contamination of the reference system by, for example, sulfides, cyanides or heavy metals
-
membrane blocked by deposits

For reliable monitoring of water quality, we recommend using electrodes from the JUMO tecLine range, for example.
Construction of a redox measurement circuit
To construct a redox measuring circuit, the following equipment components are needed:
-
immersion or flow fittings
-
metal electrode
-
reference electrode or combined metal electrode
-
shielded measuring cable
-
transducer/regulator (mV meter)
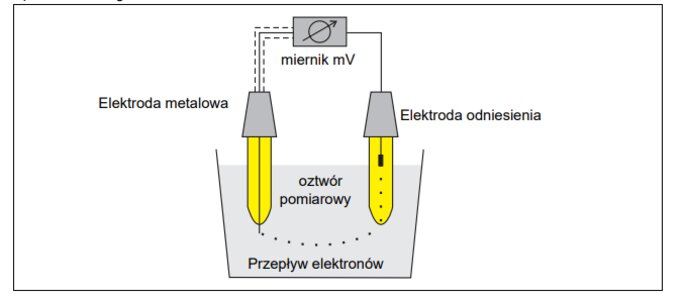
Redox measurement circuit
What is the lifetime of pH and redox sensor?
The ph measurement electrode and redox electrode wear out over time, among other things, their electrolyte is gradually diluted. The life of the electrode largely depends on the type of application and the aggressiveness of the medium under test. However, with proper storage - at room temperature, in the electrolyte and with a protective cap on - they can function properly for up to a year.

Glass pH and redox electrodes
The redox potential of swimming pool water
Pool water must undergo special treatment, and measuring the pH redox value of pool water by ph meter plays an important role. The pH value is important for flocculation, filtration and disinfection of water. For example, a pH value that is too high reduces the disinfecting effect of hypochlorite.
The redox voltage can be used to determine the bactericidal effect of pool water. Ideally, the redox voltage should be around 700mV. If the actual value falls below or significantly exceeds this value , appropriate chlorine dosing measures should be taken.
In addition, DIN 34 408 recommends the use of flow fittings for measuring redox in the water to eliminate the interfering influence of atmospheric oxygen.
- ${title}${badge}

